Asthma Treatment Gets New Breakthrough After 50 Years: How Does It Work?

Introduction
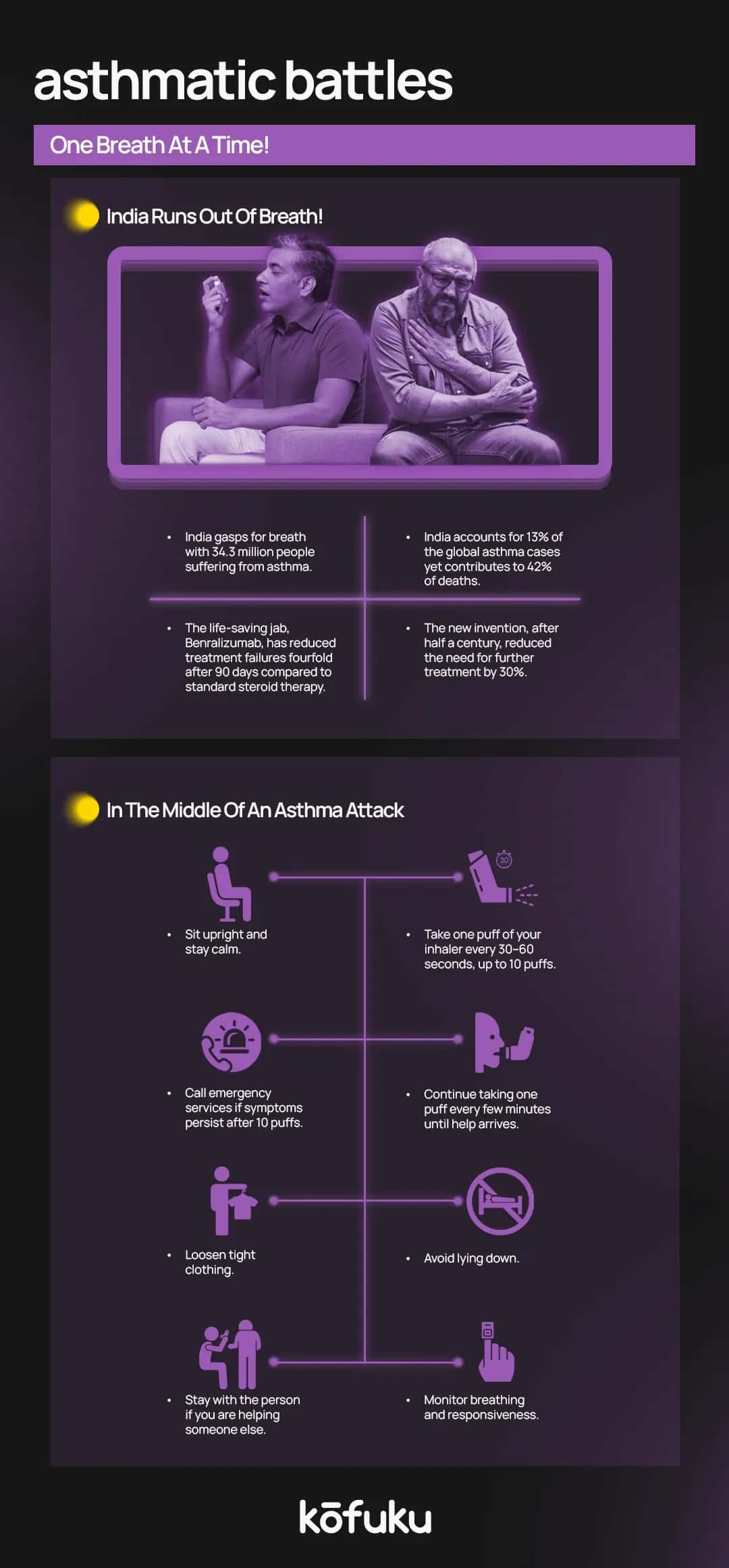
Have you ever had an asthma attack? It’s funny how we always take breathing for granted until we can’t breathe anymore. An asthma attack is a sudden worsening of asthma symptoms caused by the tightening of muscles around the airways (bronchospasm).
When an attack occurs, the lining of the airways becomes inflamed and swollen, excess mucus is produced, the air passages become narrow, and suddenly, you can’t breathe anymore.
Other symptoms of an asthma attack include intense shortness of breath, wheezing, a sudden tightness in the chest, coughing (especially at night or early morning), and an intense feeling of anxiety or panic, because you can’t breathe!
An asthma attack can be mild or life-threatening. In severe cases, speaking, breathing, or performing normal activities becomes impossible. Lips or fingernails might turn blue because of dangerously low oxygen levels.
What you need at that time is prompt treatment with a reliever inhaler (usually having a bronchodilator. If care is not administered, an asthma attack can get worse pretty quickly, resulting in hospitalisation or even the loss of life.
Over The Years
As time has gone by, asthma has seen major changes in understanding, treatment and prevalence. Early treatments focused mostly on avoiding allergens and using bronchodilators, while modern approaches incorporate inhaled corticosteroids and a proper understanding of the inflammatory nature of this ailment.
But was it always like this? Let us dial it back as far as 2600 BCE, when scriptures from China and ancient Egypt mention symptoms of breathlessness and respiratory diseases. However, asthma did not get its name or unique characteristics until Hippocrates described it more than 2,000 years later in Greece.
Hippocrates was a noted figure, often known as the “grandfather of modern medicine.” He was the first person to link asthma symptoms to environmental triggers and specific trades and professions.
The problem was that Hippocrates only saw asthma as a symptom. It wasn’t until around 100 A.C.E. that a Greek physician named Aretaeus of Cappadocia came up with a detailed definition of asthma that was quite similar to the modern understanding of how this disease develops.
His suggested remedy was to drink a concoction of owl’s blood and wine—thankfully, that is no longer a recommended intervention for asthma.
Once the Greeks were at it, could the Romans be left far behind?
In about 50 A.C.E., Pliny the Elder discovered links between pollen and breathing difficulties and was one of the first to recommend a beta2-agonist, a predecessor of epinephrine, quite well known for quick-relief asthma treatment, as a remedy for these respiratory issues.
More Recently
We couldn’t drink owl’s blood and wine for a long time. Medical technology has had to develop, and researchers and physicians have developed new approaches to asthma.
Cut to the 19th century. A doctor named Henry Hyde Salter received acclaim for his accurate descriptions and medical drawings of what happens in the lungs during asthma attacks.
He said the condition is -
“Paroxysmal dyspnoea of a peculiar character with intervals of healthy respiration between attacks”
Soon, more medical heavyweights started commenting on this. In 1892, Sir William Osler, one of the co-founders of the Johns Hopkins Medical School, developed his own definition of the disease.
Bronchial spasms were high on his list, and he noted the similarities between asthma and allergic conditions, like hay fever, as well as the tendency of asthma to run in families and begin in childhood.
According to him, there are specific triggers of asthma, like climate, diet and extreme emotion.
His focus on airway blockage due to smooth muscle spasms in the airways, instead of inflammation, meant that doctors and pharmacies started distributing medications known as bronchodilators to calm airway spasms in people with asthma.
These were widely available over-the-counter (OTC) as a treatment for asthma.
The only drawback with this method was that it had a soothing effect on a short-term basis without solving the deeper immune problems that cause asthma.
However, the public went berserk and caused an over-reliance on such medications, pushing up the number of deaths from asthma, which skyrocketed during the mid-1960s and 1980s.
A Canadian scientist, Sir William Osler, who is also called the “Father of modern medicine,” noted in his book “The Principles and Practice of Medicine” that asthma was caused by swollen bronchial membranes accompanied by spasms of the bronchial tubes. It is closely related to hay fever and is often familial and pediatric in nature.
He pinpointed nervous stimulation as a cause of an asthma attack. His research led to the idea that asthma was a psychosomatic disease. His treatment approaches were a reflection of his understanding of the nervous system triggers of asthma.
As the early 1900s began, asthma started to be treated with selective β2-adrenoceptor agonists. Belladonna alkaloids from plant sources came into focus in 1905.
Allergy immunotherapy was also introduced during the same period to treat this ailment.
Francis Rackemann found that asthma could come about for reasons other than allergy as well, and characterised allergic and non-allergic triggers of asthma in 1916.
Physicians started prescribing aminophylline suppositories and tablets and adrenaline injections for asthma in the 1940s and 1950s. Inhalation anticholinergics were also used, as were oral combinations for long-term treatment in the 1960s.
When peak flow meters were invented in the 1960s and 1970s, a technological leap bolstered effective treatment. Extensive clinical research in the 1970s resulted in the use of inhaled corticosteroids for effective asthma management.
Around 1980, we started understanding allergen exposure and the resultant release of chemical mediators that caused airway constriction. Targeted treatment options, including anti-leukotrienes, hormones, and anti-Ige therapies, emerged.
Asthma Treatment Today
Asthma today impacts over 300 million people worldwide, with 5-10% experiencing severe forms. Among these,50-6% have eosinophilic airway inflammation, a condition resistant to standard treatments like inhaled corticosteroids.
Such patients often remain symptomatic in spite of proper therapy.
Eosinophilic Asthma :
-
Eosinophils are important inflammatory cells in asthma.
-
IL-5 is important for their development and survival.
-
High eosinophil levels are linked to asthma severity, with a poor response to corticosteroids.
Phenotyping and Evaluation :
-
Picking asthma phenotypes (eg, Th-2 high vs low) helps tailor treatment.
-
Biomarkers such as FFeno and sputum eosinophil counts can help with assessment, but they have their limitations.
Current Therapies and Gaps :
-
Traditional therapies are inhaled corticosteroids, LABA, LTRAS and omalizumab.
-
Novel approaches like bronchial thermoplasty (BT) and biologics are implemented when standard therapies fail.
-
Because of treatment response variation, personalised treatment is required.

Benralizumab - A Novel Therapy
This is a humanised monoclonal antibody targeting IL-5 receptor alpha (IL-5Rα). It uniquely induces antibody-dependent cell-mediated cytotoxicity (ADCC), resulting in near-complete depletion of eosinophils. It is suitable for patients who have severe asthma and blood eosinophil counts ≥150–300 cells/μL.
Benralizumab is an anti-IL-5Rα monoclonal antibody that has shown great promise in treating eosinophilic asthma across multiple clinical trials. A Phase 1 study found rapid and continuous eosinophil depletion in blood, bone marrow, and airway tissues, going beyond the effects of other biologics like mepolizumab and reslizumab.
Phase II trials once again showcased its efficacy, especially in patients with blood eosinophil counts ≥300–400 cells/µL. These trials showed great reductions in asthma exacerbation rates and improvements in lung function and asthma control, all with a favourable safety profile.
One study highlighted benralizumab’s persistent effects after a single intravenous dose, which brought down emergency department visits and exacerbation severity for up to 24 weeks.
Because of these findings, a comprehensive Phase III program, including CALIMA, SIROCCO, and ZONDA, further cemented benralizumab’s role in severe eosinophilic asthma by reducing exacerbation frequency, improving lung function, and potentially reducing steroid-sparing effects.
Benralizumab could improve lung function and quality of life. Benefits were more obvious in people with elevated blood eosinophils, warranting further investigation in ongoing Phase III trials.

How Does This Work?
Benralizumab (MEDI-563) is a humanised IgG1k monoclonal antibody developed by AstraZeneca/MedImmune implementing hybridoma technology. It targets the interleukin-5 receptor alpha (IL-5Rα) on eosinophils, a variety of white blood cells involved in asthma and other inflammatory conditions.
The drug binds selectively to a particular amino acid residue (isoleucine-61) in domain 1 of the IL-5Rα, preventing IL-5 from binding to its receptor. This blockage disrupts signalling pathways that usually trigger eosinophil activation and survival.
Together with binding to IL-5Rα, benralizumab also binds to the FcγRIIIa ( Fc gamma receptor IIIa), also known as the Cd16a receptor. This interaction gives rise to antibody-dependent cell-mediated cytotoxicity (ADCC), which results in eosinophil apoptosis.
There are Natural Killer cells that release proapoptotic proteins, such as perforins and granzymes, that promote the destruction of eosinophils.
The standout feature of bevacizumab is its superior affinity for FcγRIIIa because of the absence of fucose molecules on the antibody. This boosts its effectiveness in inducing ADCC by 5 to 50 times compared to other monoclonal antibodies.
The twin action method of blocking IL-5 signalling and enhancing eosinophil apoptosis through NK cells makes benralizumab quickly and effectively deplete eosinophils in patients with eosinophilic asthma. This reduction in eosinophil counts in the airways and peripheral blood results in a marked decrease in inflammation and better asthma control.
Thus, this powerful and targeted approach is a landmark advancement in the treatment of severe eosinophilic asthma, especially for patients who don’t do too well with traditional therapies.
Conclusion
After years of getting nowhere in terms of asthma treatment, the arrival of Benralizumab is a breakthrough moment in asthma care, especially for those having severe eosinophilic asthma who suffer despite standard treatments.
Benralizumab, through its unique mechanism, does not directly target and deplete eosinophils. It also reduces exacerbations and improves lung function and quality of life. Strong clinical trial data back it up, and it is a testament to a shift towards personalised, biomarker-driven therapy in respiratory medicine.
As research carries on, Benralizumab is a beacon of hope-offering long-awaited relief to patients and changing how we approach the treatment of complex, inflammatory airway ailments like asthma.
FAQs
Q. What is eosinophilic asthma, and how is it different from regular asthma?
A. Eosinophilic asthma is a severe form of asthma where eosinophils, a type of white blood cell, primarily cause the inflammation in the airways. This form of asthma often does not respond well to standard treatments.
Q. How does benralizumab work to treat severe eosinophilic asthma?
A. Benralizumab is a monoclonal antibody that targets the IL-5 receptor alpha (IL-5Rα) on eosinophils. It induces cell death in eosinophils, significantly reducing eosinophil levels, which helps reduce inflammation and asthma symptoms in patients.
Q. What are the clinical benefits of using benralizumab in asthma treatment?
A. Clinical trials have shown that benralizumab significantly reduces asthma exacerbations, improves lung function, and provides a better quality of life for patients, particularly those with high eosinophil counts.
Q. How does benralizumab compare to other biologics used for asthma treatment?
A. Compared to biologics like mepolizumab and reslizumab, benralizumab shows more rapid and sustained eosinophil depletion, leading to better asthma control.
Q. Who is a suitable candidate for benralizumab treatment?
A. Benralizumab is most suitable for patients with severe eosinophilic asthma, especially those with blood eosinophil counts of ≥150–300 cells/μL. It is an option for individuals who do not respond well to standard asthma treatments.

The History, Impact, and Importance of Vaccines Worldwide

What You Need to Know About the Russian Cancer Vaccine

10 Simple Steps to Prevent Common Lifestyle Diseases

10 Things to Do at Home During an Asthma Attack

Everything You Need To Know About Stress-Induced Asthma


